Advancing Innovation Through Research
At Chicago Cosmetic Surgery and Dermatology, our dedicated research team is committed to pushing the boundaries of cosmetic surgery and dermatology. Every study we conduct follows Good Clinical Practice (GCP) guidelines and FDA regulations, ensuring patient safety and the highest standard of care remain at the center of everything we do.
Clinical Trials That Shape the Future
Since launching our research program, we’ve focused on clinical trials that explore the latest in injectables and advanced device technologies. These studies target a range of conditions, from cellulite reduction and the treatment of active facial lines to abdominal muscle restoration in postpartum women.
Our portfolio includes investigator-initiated studies, sponsor-driven projects, and FDA-monitored trials—all reflecting our mission to advance treatment options, improve patient outcomes, and redefine what’s possible in aesthetics.
Current Research Studies
Explore our Current Research Studies, where we’re actively recruiting participants to help advance the future of cosmetic surgery and dermatology while receiving access to innovative treatments under expert care.
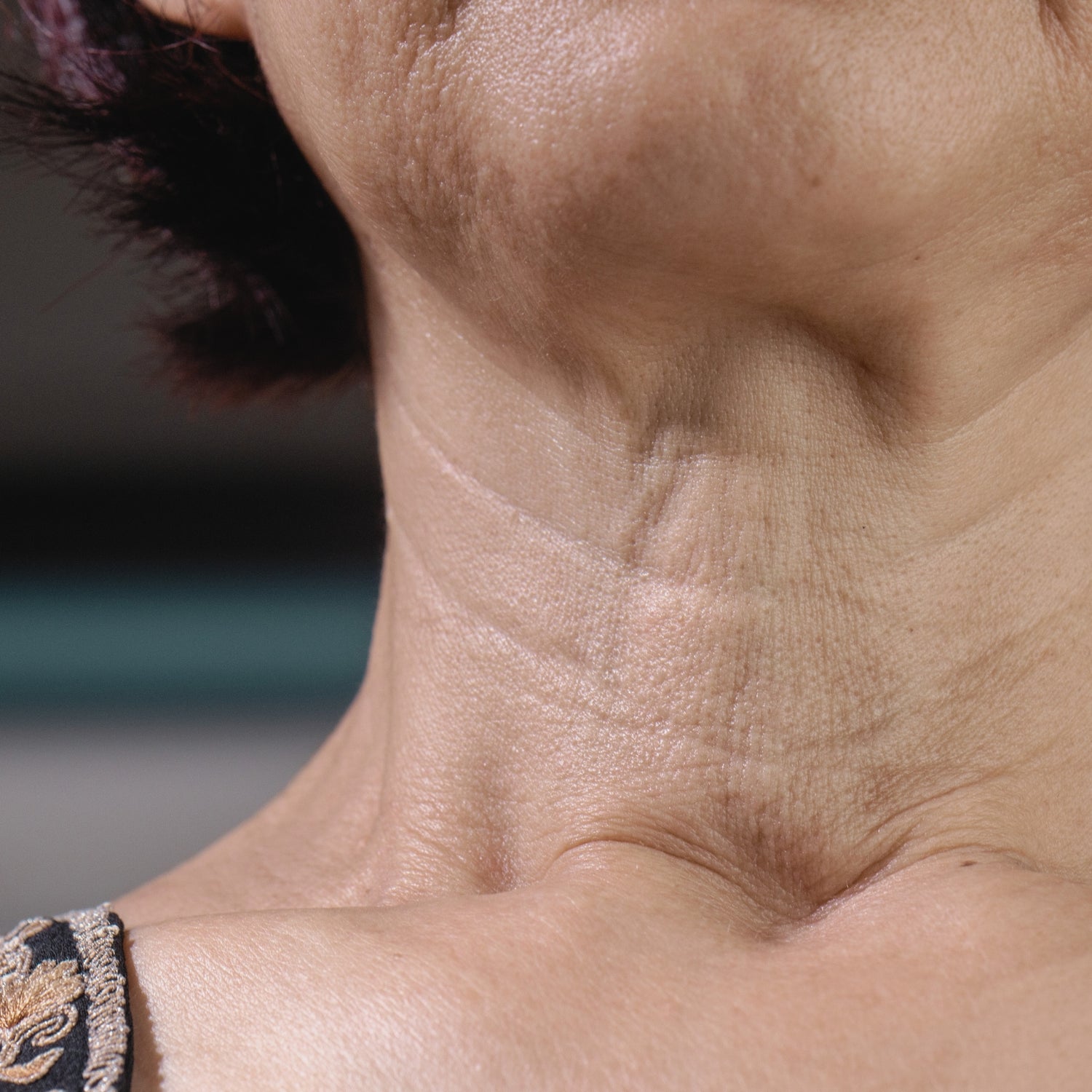
Bausch – Thermage
Purpose: Study to evaluate the safety and effectiveness of Thermage FLX Radiofrequency Treatment to improve lines and wrinkles and to life and tighten lax tissue in the neck, abdomen, upper arms, and face.
Criteria: Male or Female, 30 to 60 years of age, Have mild to moderate skin laxity of the neck, abdomen, upper arms and/or face, mild to moderate lines/wrinkles of the neck and face, BMI ≤30. Cannot have an implant in treatment zone. Cannot have had 1 or more prior cosmetic procedure in the target treatment zone within 6-12 months (depending on the procedure), tattoos in the treatment zone. (Other criteria may apply)
Number of Visits: 4 visits – Screening Visit, Baseline (Day 1), Day 90, Day 180.

Ourself – Investigational Face Serum
Purpose: Ourself is conducting a 12-week clinical research study to evaluate the effects of an investigational face serum on improving overall facial skin quality. The study product may improve the appearance of your skin, but the results cannot be guaranteed.
Criteria: You may qualify if you:
- Are a healthy female or male between 35-66 years old
- Have at least two of the following mild to severe skin concerns on the facial area:
- Fine lines/wrinkles
- Radiance/brightness
- Plumpness (loss of volume)
- Elasticity
- Skin roughness
- Sagging
- Overall skin quality/appearance
Participation Requirements:
- Use only the study-provided Test Product and Investigator-approved skincare regimen during the study (up tp 4 months).
- Avoid starting any new makeup products during the study (up to 4 months).
- Avoid undergoing any cosmetic procedures on your face during the study (up to 4 months).
- Be available for up to 6 office visits during the study period.
- Agree to have facial photographs taken and sign a release allowing Ourself, Inc. to use these images for promotional purposes.
- Meet additional study qualification criteria.
Upcoming Research Studies
Stay tuned for our Upcoming Research Studies, offering new opportunities to participate in groundbreaking trials that shape the future of cosmetic surgery and dermatology. *Start dates are approximate. Final dates may vary.
Research Study Inquiry Form
Why choose Chicago Cosmetic Surgery & Dermatology?





FAQ's for Research Studies
What are clinical research studies?
What are clinical research studies?
Clinical research studies, also known as clinical trials, are carefully designed medical investigations that test new treatments, devices, or procedures to ensure they are safe and effective.
Why should I participate in a research study?
Why should I participate in a research study?
By joining a study, you not only gain access to innovative treatments before they are widely available, but you also play a vital role in advancing the future of cosmetic surgery and dermatology.
Are research studies safe?
Are research studies safe?
Yes. All of our studies follow strict Good Clinical Practice (GCP) guidelines and FDA regulations. Patient safety is always our top priority, and our providers monitor each participant closely throughout the study.
Who can participate in a study?
Who can participate in a study?
Eligibility varies depending on the type of trial. Each study has specific requirements related to age, health history, or treatment goals. Our team will guide you through the screening process to see if you qualify.
What types of studies are offered at Chicago Cosmetic Surgery and Dermatology?
What types of studies are offered at Chicago Cosmetic Surgery and Dermatology?
We conduct a wide range of studies, from new injectable treatments and advanced device trials to procedures targeting cellulite, facial lines, and muscle restoration after pregnancy.
How do I find out about current or upcoming studies?
How do I find out about current or upcoming studies?
You can view our list of current and upcoming studies on this page or contact our research team directly for more information about how to get involved.
Will I get paid for the studies?
Will I get paid for the studies?
While some studies offer participant payment (amount varies), most are not. However, any products or treatments utilized during the study are offered to you at no cost.
Take the first step to feel great in your own skin.
Get started with a consultation and find out what our team of experts can do for you.




